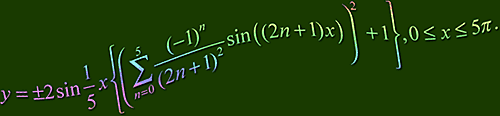|
SCI-FUN's Christmas Card
|
|
Inside-right
|
||||||||
|
|
||||||||
|
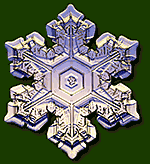
Real Snowflakes
|
||||||||
|
||||||||
|
||||||||
|
|
||||||||
|
Holly and Berries #1
|
||||||||
The mathematical berries at bottom right are three polyhedra (the images for which are from the mathworld site, coloured red for Christmas). The first is the intersection of five octahedrons (solids made from eight equilateral triangles); the second is the intersection of a dodecahedron (made from ten pentagons) and an icosahedron (constructed from twenty equilateral triangles); the third is made from twenty rhombuses [mathworld prefers 'rhombi']. Click on the compound names below for more information on each solid, including an animated version (or on the thumbnails themselves for a larger image).
|
||||||||
|
Mathematical Holly
|
||||||||
|
If you haven't worked it out already (you mean you haven't tried? grrr) then click here or the image above for a description of the mathematical equation...
|
||||||||
|
|
||||||||
|
Möbius Paper Chains
|
||||||||
 |
||||||||
|
It's not simply a mathematical curiosity. In the early 1970s, a möbius conveyor belt was built which minimised wear by using 'both' surfaces of the rubber. And some tape loops were designed in this way to double the recording time! A variety of molecules have also been synthesised with möbius properties, although (as far as we're aware) no specific chemical benefits result from the structure. There's a nice Java animation of a mobius strip at this mathworld page. |
||||||||
|
|
||||||||
| If you cut a normal paper link down the middle, you separate it into two individual rings. If you cut the möbius strip in this way, would you end up with two möbius strips, half the width? Try it. (And if you think you know the answer to that one, ask yourself what would happen if your cut didn't go down the middle of the band, but instead split the width into one quarter and three quarters, for example.) | ||||||||
|
|
||||||||
|
|
||||||||
|
Holly and Berries #2
|
||||||||
| The decoration at top-right is more straightforward than the mathematics one: it's some strands of DNA (not, alas, any sequences that are found in real holly, unless by sheer coincidence :-), surrounding three buckyballs, which are... | ||||||||
|
Buckminsterfullerenes
|
||||||||
|
And then (as described here) Curl, Kroto and Smalley discovered a new form, a stable molecule with sixty atoms... The only way in which these atoms could combine was as the football shape shown opposite, which exactly mirrors the old-style leather footballs, mixing hexagonal and pentagonal panels. Because this structure was also found in the design of a geodesic dome built by the architect Buckminster Fuller, the molecule was named buckminsterfullerene,* usually shortened to fullerene (or buckyball). Fullerenes exhibit some remarkable properties, such as superconductivity; indeed, they may prove to superconduct at higher temperatures than the best of today's ceramic materials. By attaching other atoms to the carbons, a fuzzyball can be created; materials made from fullerenes attached to fluorine atoms will (it's believed) produce a slicker compound than teflon, currently the solid with the lowest coefficient of friction. Other atoms can be trapped inside fullerenes (a bit like our logo above); recent evidence for a meteor impact at the end of the Permian period was found by analysing noble gases preserved within the molecule. One avenue of research involves using fullerenes to help in the targeting of drugs within the body. "The buckyball, being the roundest of round molecules, is also quite resistant to high speed collisions. In fact, the buckyball can withstand slamming into a stainless steel plate at 15,000 mph, merely bouncing back, unharmed. When compressed to 70 percent of its original size, the buckyball becomes more than twice as hard as its cousin, diamond." – from "The Buckyball", a fascinating article by Rodrigo de Almeida Siqueira. |
||||||||
| Nanotubes are essentially one-dimensional fullerenes, a convex cage of atoms with only hexagonal and/or pentagonal faces (see below). Nanotubes exhibit extraordinary strength (they are the strongest fibres known), with the potential to revolutionise the science of material composites, and have fascinating electrical properties, which may prove important in future microelectronics, at scales smaller than today's silicon technology. (See Carbon Nanotube Electronics for more information.) | ||||||||
|
There have recently been concerns raised (sensible ones, as opposed to Prince Charles type, Michael Crichton-inspired 'grey goo' worries) that nanoparticles may pose considerable environmental hazards. The physical properties – and potential biotoxicity – of nanometre-scale non-biodegradable objects are currently under investigation. The Nanotechnology Now site has extensive information (and a huge section of links) on fullerenes and nanotubes. * Of course, it's possible – if all the discoverers had been British (and avid football fans) – that the molecule might have ended up being known as footballene, or, god forbid, davidbeckhamene... |
||||||||